LAB HOURS: MON - THU and SAT : 9:00 AM to 9:00 PM
FRI and SUN : 9:00 AM to 6:00 PM

Accreditation according to ISO 15189
FML at Arab Health 2011, Lecture in 'Lab Management'

Medical Diagnostic Laboratories: Accreditation according to ISO 15189
Associate Professor Michaela Jaksch, MD PhD
Freiburg Medical Laboratory ME LLC, Dubai
Abstract
For medical diagnostic laboratories, accreditation and quality standards such as CPA (Clinical Pathology Accreditation, United Kingdom), CAP (College of American Pathologists; worldwide and mainly USA) or ISO 15189 (mainly Europe) as well as many others exist. Accreditation allows us to make an informed decision when selecting a laboratory, as it demonstrates competence, impartiality and capability. It helps to increase confidence in credibility and performance of services. Accreditation bodies around the world, which have been evaluated as competent, have signed an agreement that enhances the acceptance of products and services across national borders. The purpose of this arrangement, the International Laboratory Accreditation Cooperation (ILAC) Arrangement, is to create an international framework and to remove technical barriers (www.ilac.org). ILAC denotes the collaboration of accreditation bodies that accredit calibration laboratories, testing laboratories and/or inspection bodies as well as medical laboratories. The ILAC network currently consists of 135 bodies representing 88 different economies. Current standards recognized as meeting the accreditation requirements of ILAC, for inclusion in the ILAC Mutual Recognition Arrangement include: ISO/IEC 17025:2005 general requirements for the competence of testing and calibration laboratories and ISO 15189:2007 medical laboratories – particular requirements for quality and competence medical testing laboratories
The ISO 15189 standard, designed specifically for medical laboratories, covers 15 management requirements and 8 technical requirements. The standard is concise and to the point and is now part of requirements in other standards, such as CPA or CAP. In brief, the organization and management requirements are: quality management system, document control, review of contracts, examination by referral laboratories, external services and supplies, advisory services, resolution of complaints, identification and control of nonconformities, corrective action, preventive action and continual improvement. The technical requirements include educated personnel, accommodation and environmental conditions, laboratory equipment, pre-examination procedures, examination procedures, assuring quality of examination procedures, post-examination procedures and reporting of results.
The lecture gives an overview on the accreditation according to ISO 15189 in the worldwide context with special focus on requirements in the UAE (www.dac.dm.ae).
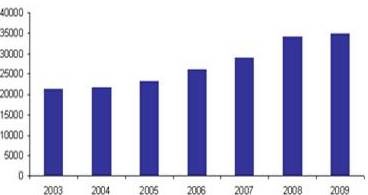
Total Number of Accredited Labs Worldwide (ILAC)
Learning Objectives:
International Accreditation
ISO 15189
UAE Recommendation: Accreditation for Medical Facilities
Advantages and Disadvantages in Accreditation

Lab hours
MON - THU and SAT 9:00 AM to 9:00 PM | FRI and SUN : 9:00 AM to 6:00 PM













